Reaction kinetics and a physical model of the charring layer by depositing Al2O3 at ultra-high temperatures
Abstract
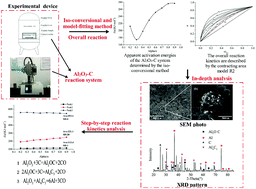
The thermochemical ablation of insulation material caused by slag deposition in solid rocket motors has increasingly attracted researchers’ attention. Understanding the ablation mechanism and the ability to calculate reaction kinetics parameters determine the height of the thermal protection design for advanced solid rocket motors. In this work, the interaction of the Al2O3–C system is determined through static ablation experiments. Using X-ray diffraction, HSC thermodynamic software, and a thermogravimetric analyser, the carbon thermal reduction of alumina is analysed and the reaction mechanism and physical model are obtained. Isothermal experiments at 1700–1850 °C and mathematical analysis provide the kinetic parameters of the overall and step-by-step reactions. The results show that the overall reaction of the Al2O3–C system involves three steps. The overall reaction kinetics are described by the contracting area model R2 with apparent activation and frequency factors estimated as 254.5 kJ mol−1 and 5.5 × 106 min−1, respectively. The distribution reaction kinetics of steps 1 and 2 are described by the first-order chemical reaction control model (F1) and that of step 3 is described by the one-dimensional diffusion control model (D1). The corresponding activation energies are 107.9 kJ mol−1, 240.3 kJ mol−1, and 567.5 kJ mol−1, and frequency factors are 625.94 min−1, 8.3 × 105 min−1, and 1.6 × 1014 min−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...