The effect of surface coverage on N2, NO and N2O formation over Pt(111)†
Abstract
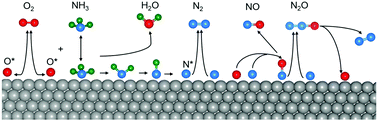
The effect of surface coverage of species, θ, on the kinetic parameters of N2, NO and N2O formation in a system simulating ammonia oxidation over Pt(111) has been studied by using periodic density functional theory (DFT). The energy barriers for product formation decrease as θ increases, with the effect being more significant above 0.25 ML. The heat of surface reaction decreases as θ increases, making the dissociation of the products less favourable due to a weaker interaction of the intermediates with the surface. The effect of θ on the binding energy is stronger for N* than for either O* or NO*, but it is more apparent in the co-adsorption with O* and NO*. Similarly, the coverage of N* more strongly affects the activation energy of N2 and N2O desorption than does the coverage of O* for NO* and N2O formation.


 Please wait while we load your content...
Please wait while we load your content...