Nanoparticle-induced ion-sensitive reduction in decane–water interfacial tension†
Abstract
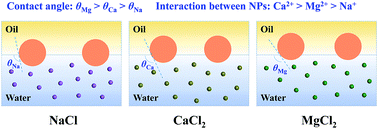
The synergistic effect of ions and nanoparticles on the interfacial tension is of great significance for extensive applications in interface-related industrial processes. However, its mechanisms are still unclear owing to a lack of understanding on the interaction between nanoparticles/ions at the interface. Here, we employ the molecular dynamics method to explore the synergistic effect of ions and nanoparticles on reducing the decane–water interfacial tension and reveal the dominant role of the three-phase contact angle and the interaction between nanoparticles. The results show that the reduction of interfacial tension is sensitive to cation species and temperature. The stronger hydration of cations induces an increased three-phase contact angle, weakening the interaction between nanoparticles and water molecules at the interface. Hence, the virial term of interfacial tension decreases. Meanwhile, the potential of mean force between nanoparticles at the interface indicates that the order of interaction strength between nanoparticles for different cations is Ca2+ > Mg2+ > Na+. The strong interaction between nanoparticles restricts the motion of nanoparticles and water molecules at the interface, inducing a reduced kinetic energy term of interfacial tension. Therefore, the interfacial tension decreases after adding the nanoparticles. Besides, as temperature rises, the difference in the adsorption ability of nanoparticles on water molecules causes a falling interfacial tension with a characteristic stage.


 Please wait while we load your content...
Please wait while we load your content...