Protonation-induced molecular permeation at the oil/water interface in an electric field†
Abstract
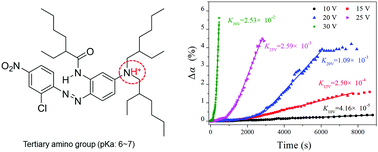
As a common physicochemical phenomenon, protonation can cause molecules, atoms or ions with lone-pair electrons to become charged, and can further cause some changes in their physical and chemical properties. Our study first focused on the molecular protonation process and accompanying transitions of the oil/water interface properties in an electric field. The relationship between the protonation degree increment and applied voltage was proposed as a guide for controlling the protonation via applying an electric field. Besides the protonation degree, the water solubility of the oily target molecule obviously increased at 30 V for 600 s along with electric field-driven protonation. At the same time, the electrical conductivity and the underwater interface wettability of oil phase transitioned. These property transitions are anticipated to guide the further improvement and updating of promising protonation functions.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...