Central substitution of azacalixphyrins: a strategy towards acidochromic NIR dyes†
Abstract
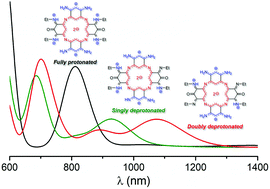
Azacalixphyrin derivatives constitute one of the most intriguing class of macrocyclic compounds. Indeed, these isostructural and isoelectronic analogues of porphyrins intensively absorb light up to the near infrared region, exist in several tautomeric forms and present a bis-zwitterionic structure, with a central dianionic core surrounded by positively-charged trimethine cyanines. However, control of the position of the absorption bands of azacalixphyrin remains an important challenge, as the experimental attempts reported to date have led to very modest auxochromic shifts only. Inspired by previous work demonstrating that the optical signatures of cyanines can be strongly modified by using central substituents, we have evaluated the validity of this strategy for azacalixphyrin considering several substituents positioned in symmetric or asymmetric manners around the core and linked through both single and double bonds, as well as several protonation states of the macrocycles. It turns out that bromine and dimethylamino substituents have a negligible or weak impact on the optical properties of azacalixphyrins with maximal redshifts smaller than 0.10 eV. The imino substitution induces strong geometrical deformations that counterbalance the electronic effects leading to rather modest variations of the optical signatures. In contrast, for keto-substituted macrocycles, electronic effects dominate and very strong acidochromic shifts are predicted with absorption wavelengths going from 811 to 1095 nm upon double deprotonation.


 Please wait while we load your content...
Please wait while we load your content...