Reactivity and energy level of a localized hole in liquid water
Abstract
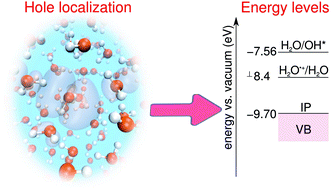
We study the reactivity and the energy level associated with the reduction of the H2O˙+ radical cation in liquid water by combining ab initio molecular dynamics simulations at the hybrid functional level, a grand-canonical formulation of solutes in aqueous solution, and nudge-elastic-band calculations. We demonstrate that this extremely oxidative solute promptly dissociates and calculate an energy barrier for the reaction of 0.06 eV, consistent with the short measured lifetimes. We calculate the energy level related to the H2O˙+/H2O redox couple with respect to the vacuum level and to the computational standard hydrogen electrode (SHE), using the thermodynamic integration method. This energy level is found to lie at 3.8 ± 0.1 eV above the SHE level, in remarkable agreement with a previous estimate based on thermodynamical data. The implications of the present results for the mechanism of water splitting at the heterogenous semiconductor–water interface are discussed.


 Please wait while we load your content...
Please wait while we load your content...