Tracking the interfacial charge transfer behavior of hydrothermally synthesized ZnO nanostructures via complementary electrogravimetric methods†
Abstract
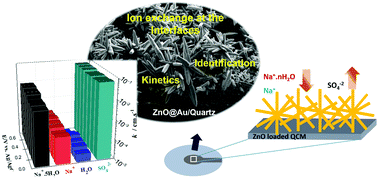
The mechanism of species fluxes during the charge–discharge process in a nanostructured ZnO electrode was studied by a combined methodology of electrochemical quartz-crystal microbalance (EQCM) and ac-electrogravimetry. Under the conditions of this study, anions (SO42−) possess the highest kinetics to be transferred at the electrode/electrolyte interface in the charge balance while cations (identified as Na+·5H2O and Na+) play the major role as charge carriers. Free H2O molecules present a sluggish behavior and their interfacial transfer occurs at a low scan rate or low frequencies. These findings shed light on the nature of ions and solvent participation in the charge balance of hydrothermally synthesized ZnO nanostructures directly grown on a QCM device. The combined methodology proposed herein provides dynamic and gravimetric analysis of interfacial charge transfer and can be extended to investigate other nanostructured metal oxide-based electrodes for energy storage.


 Please wait while we load your content...
Please wait while we load your content...