Solvation and hydrogen bonding aided efficient non-radiative deactivation of polar excited state of 5-aminoquinoline†
Abstract
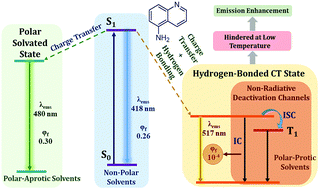
The fluorescence of 5-aminoquinoline (5AQ) is quenched strongly in alcoholic solvents, in stark contrast to the enhancement in fluorescence observed earlier for its positional isomer, 3-aminoquinoline (3AQ). This phenomenon has been explained by the involvement of a highly dipolar excited state, which is manifested in the solvatochromism of 5AQ. A marked wavelength dependence of fluorescence decays of 5AQ in alcoholic solvents is ascribed to the ultrafast solvation of the highly dipolar excited state in these solvents. The resultant stabilization of this state leads to a decrease in the gap between its energy and lower lying triplet as well as ground singlet states, resulting in an efficient non-radiative relaxation and consequently, fluorescence quenching. Solvation dynamics reported for 5AQ is somewhat slower than earlier reports with coumarin probes, due to the involvement of solute–solvent hydrogen bonds, especially in the excited state of the solute. At lower temperatures, solvation is slowed down. An extreme case of this phenomenon is the absence of solvent relaxation at liquid nitrogen temperature. The fluorescence lifetime increases from tens of picoseconds at room temperature to tens of nanoseconds at cryogenic temperature, lending credence to the proposed model.
- This article is part of the themed collections: New Frontiers in Indian Research and New Frontiers in Indian Research


 Please wait while we load your content...
Please wait while we load your content...