Experimental and theoretical investigations of infrared multiple photon dissociation spectra of arginine complexes with Zn2+ and Cd2+†
Abstract
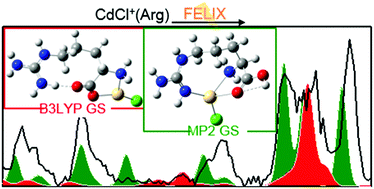
Arginine (Arg) complexes with Zn2+ and Cd2+ were examined by infrared multiple photon dissociation (IRMPD) action spectroscopy using light from a free electron laser. Electrospray ionization generated complexes of deprotonated Arg with Zn2+, [Zn(Arg–H)]+, and Arg with CdCl+, CdCl+(Arg). Possible low-energy conformers of these species were found using quantum chemical calculations, and their calculated IR spectra were compared to experimentally measured IRMPD spectra. Calculations were performed at the B3LYP/6-311+G(d,p) level for Zn2+ complexes and B3LYP/def2-TZVP with an SDD effective core potential on cadmium for CdCl+ complexes. [Zn(Arg–H)]+ was found to adopt a charge-solvated, tridentate [N,CO−,Nω′] structure where Zn2+ binds to the backbone amine, carbonyl oxygen, and side-chain terminal guanidine nitrogen (Nω′). The CdCl+(Arg) species was suggested to be a mixture of a dominant (∼85%) charge-solvated, tridentate [N,CO,Nω′] structure where the CdCl+ binds to the backbone amine, carbonyl, and side-chain imine (Nω′) and a minor (∼15%) bidentate [N,CO−](Nω′H2+) zwitterionic structure where the metal center binds to the backbone amine and carbonyl oxygen with intramolecular proton migration from the hydroxyl to the Nω′ guanidine nitrogen (as designated in parenthesis).


 Please wait while we load your content...
Please wait while we load your content...