Tubular J-aggregates of a new thiacarbocyanine Cy5 dye for the far-red spectral region – a spectroscopic and cryo-transmission electron microscopy study†
Abstract
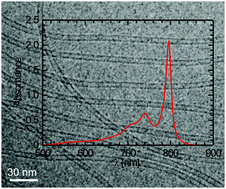
The aggregation behavior of a phenol-substituted thiacarbocyanine Cy5 dye (5-chloro-2-[5-[5-chloro-3-(4-sulfobutyl)-3H-benzothiazol-2-ylidene]-3-phenyl-penta-1,3-dienyl]-3-(4-sulfobutyl)-benzothiazol-3-ium hydroxide, inner salt, triethylammonium salt) in aqueous solution is investigated using steady-state absorption, linear dichroism, and fluorescence spectroscopies, as well as cryogenic transmission electron microscopy (cryo-TEM). By increasing the concentration, the dye self-assembles in pure water into dimers and H-aggregates, the latter being uniform particles of ∼2.6 nm size. In the presence of NaCl, two different types of J-aggregates are observed depending on salt concentration (varied from 10 to 100 mM). At low salt concentration (10 mM) a J-aggregate of extended mono-layered sheets prevails, which disappears after a few days, whereas a second type of J-aggregate emerges. Generally, the latter dominates in matured solutions in particular at high salt concentration and seems to be the thermodynamically stable species. This J-aggregate shows three perpendicularly polarized absorption bands and fluoresces in the far-red at around 800 nm. The most intensive and very narrow (fwhm of 238 cm−1) absorption band is centered at 796 nm. Cryo-TEM reveals uniform nanotubes of ∼7 nm diameter and micrometer length. They represent the first tubular cyanine dye J-aggregates that are active in the far-red. Moreover, the studied dye is a prime example of cyanine dyes showing two self-assembly pathways that lead to different species of J-aggregates with distinct optical and morphological properties.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...