Hexagonal Ti2B2 monolayer: a promising anode material offering high rate capability for Li-ion and Na-ion batteries†
Abstract
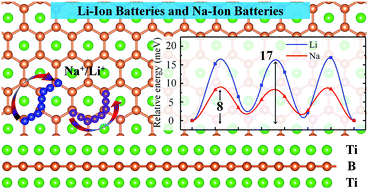
Combining the first-principles density functional method and crystal structure prediction techniques, we report a series of hexagonal two-dimensional transition metal borides including Sc2B2, Ti2B2, V2B2, Cr2B2, Y2B2, Zr2B2, and Mo2B2. Their dynamic and thermal stabilities are testified by phonon and molecular dynamics simulations. We investigate the potential of the two-dimensional Ti2B2 monolayer as an anode material for Li-ion and Na-ion batteries. The Ti2B2 monolayer possesses high theoretical specific capacities of 456 and 342 mA h g−1 for Li and Na, respectively. The very high Li/Na diffusivity with an ultralow energy barrier of 0.017/0.008 eV indicates an excellent charge–discharge capability. In addition, good electronic conductivity during the whole lithiation process is found by electronic structure calculations. The very small change in volume after the adsorption of one, two, and three layers of Li and Na ions indicates that the Ti2B2 monolayer is robust. These results highlight the suitability of Ti2B2 monolayer as well as the other two-dimensional transition metal borides as excellent anode materials for both Li-ion and Na-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...