Li doped kagome spin liquid compounds†
Abstract
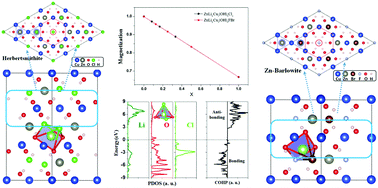
Herbertsmithite and Zn-doped barlowite are two compounds for experimental realization of two-dimensional kagome spin liquids. Theoretically, it has been proposed that charge doping a quantum spin liquid gives rise to exotic metallic states, such as high-temperature superconductivity. However, one recent experiment on herbertsmithite with successful Li-doping surprisingly showed an insulating state even under a heavily doped scenario, which cannot be explained by previous theories. Using first-principles calculations, we performed a comprehensive study on the Li intercalation doping effect of these two compounds. For the Li-doped herbertsmithite, we identified the optimized Li position at the Cl–(OH)3–Cl pentahedron site instead of the previously speculated Cl–(OH)3 tetrahedral site. With increasing Li doping concentration, saturation magnetization decreases linearly due to charge transfer from Li to Cu ions. Moreover, we found that Li forms chemical bonds with nearby (OH)− and Cl− ions, which lowers the surrounding chemical potential and traps electrons, as evidenced by the localized charge distribution, explaining the insulating behavior measured experimentally. Though a different structure from herbertsmithite, Zn-doped barlowite shows the same features upon Li doping. We conclude that Li doping this family of kagome spin liquids cannot realize exotic metallic states, and other methods should be further explored, such as element substitution with those having different valence electrons.


 Please wait while we load your content...
Please wait while we load your content...