Experimental and theoretical insights into the influence of electronic density on proton-transfer reactions†
Abstract
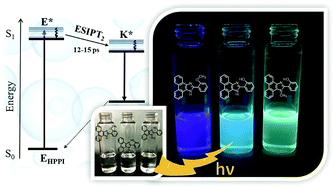
We report on the excited-state behavior of proton-transfer phenanthroimidazole derivatives, such as HPPI and NMHPPI, in solutions using steady-state and femto- to nanosecond time-resolved fluorescence spectroscopies. Experimental observations are supported by theoretical calculations (TDDFT). In dichloromethane (DCM) and acetonitrile (ACN), two different paths are found for the excited-state intramolecular proton-transfer (ESIPT) reactions following two different channels. A fast and direct channel (ESIPT1) in 1–2.5 ps and a slower one (ESIPT2) in 12–15 ps, the latter being more influenced by the solvent viscosity (30 ps for HPPI and 20 ps for NMHPPI in triacetin (TAC) solutions). The slowing down of the ESIPT2 reaction is explained in terms of an intramolecular charge transfer (ICT) reaction coupled to a twisting motion to reach a more suitable conformation of the involved parts in the proton-transfer motion. The absence of OH/OD exchange effects in the ultrafast and slow proton-transfer dynamics suggests that the ESIPT reactions, which involve intramolecular and solvent coordinates, do not occur via tunneling. These results reveal new insights into the photobehavior of proton-transfer dyes, which might help in designing photosensors or lighting devices.


 Please wait while we load your content...
Please wait while we load your content...