Fe–V sulfur clusters studied through photoelectron spectroscopy and density functional theory†
Abstract
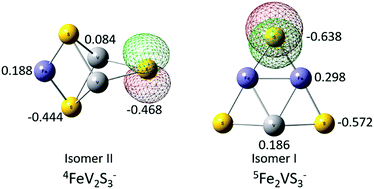
Iron–vanadium sulfur cluster anions are studied by photoelectron spectroscopy (PES) at 3.492 eV (355 nm) and 4.661 eV (266 nm) photon energies, and by density functional theory (DFT) calculations. The structural properties, relative energies of different structural isomers, and the calculated first vertical detachment energies (VDEs) of different structural isomers for cluster anions FeVS1–3− and FemVnSm+n− (m + n = 3, 4; m > 0, n > 0) are investigated at a BPW91/TZVP theory level. The experimental first VDEs for these Fe–V sulfur clusters are reported. The most probable ground state structures and spin multiplicities for these clusters are tentatively assigned by comparing their theoretical and experiment first VDE values. For FeVS1–3− clusters, their first VDEs are generally observed to increase with the number of sulfur atoms from 1.45 eV to 2.86 eV. The NBO/HOMOs of the ground state of FeVS1–3− clusters are localized in a p orbital on a S atom; the partial charge distribution on the NBO/HOMO localized site of each cluster anion is responsible for the trend of their first VDEs. A less negative localized charge distribution is correlated with a higher first VDE. Structure and steric effect differences for FemVnSm+n− (m + n = 3, m > 0, n > 0) clusters are suggested to be responsible for their different first VDEs and properties. Two types of structural isomers are identified for FemVnSm+n− (m + n = 4, m > 0, n > 0) clusters: a tower structure isomer and a cubic structure isomer. The first VDEs for tower like isomers are generally higher than those for cubic like isomers of FemVnSm+n− (m + n = 4, m > 0, n > 0) clusters. Their first VDEs are can be understood through: (1) NBO/HOMO distributions, (2) structures (steric effects), and (3) partial charge numbers on the NBO/HOMO's localized sites. EBEs for excited state transitions for all Fe–V sulfur clusters are calculated employing OVGF and TDDFT approaches at the TZVP level. The OVGF approach for these Fe/V/S cluster anions is better for the higher transition energies than the TDDTF approach. The experimental and theoretical results for these Fe/V/S cluster anions are compared with their related pure iron sulfur cluster anions. Properties of the NBO/HOMO are essential for understanding and estimating the different first VDEs for Fe/V/S, and comparing them to those of the pure Fe/S cluster anions.


 Please wait while we load your content...
Please wait while we load your content...