Direct evidence for surface long-lived superoxide radicals photo-generated in TiO2 and other metal oxide suspensions†
Abstract
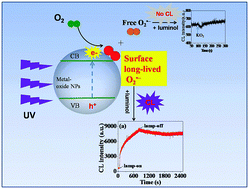
Heterogeneous catalytic reactions usually proceed at the surfaces of materials, where many intermediates, such as free radicals, usually were believed to be short-lived. Herein, surface long-lived superoxide radicals (O2˙−) were identified in UV-irradiated aqueous suspensions of TiO2 and other metal oxide nanoparticles using an online chemiluminescence system. From the decay dynamics process of O2˙−, a long-lived O2˙− radical was observed on anatase TiO2 at pH = 12. After separation of the photo-excited suspension via filtration, CL was detected from the particles but not the filtrate, thus confirming O2˙− surface adsorption. The unusual stability of O2˙− was also verified using density functional theory (DFT) calculations. The lifetimes of the radicals were estimated on the different kinds of semiconductor surface according to the decay dynamics curves, and followed the order: TiO2 > ZnO > SnO2 > CeO2 > Fe2O3. Furthermore, the function of surface long-lived O2˙− in TiO2 suspensions with regards to photochemical conversion was investigated using NBT as a chemical model; it was found that half of the molecules were reduced by the surface-adsorbed O2˙−. The finding of surface-stabilized, long-lived superoxide radicals may have important implications in relation to the chemistry, biology and toxicology of these radicals.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...