Effect of density on the thermal decomposition mechanism of ε-CL-20: a ReaxFF reactive molecular dynamics simulation study
Abstract
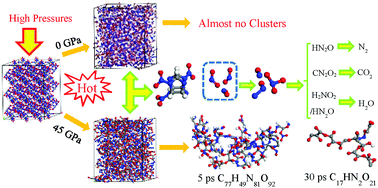
The explosive detonation reaction occurs when explosives are compressed by different shock strengths, and the degree of compression affects the chemical reaction of the detonation process. The thermal decomposition mechanism of explosives under different compression densities has thus attracted significant research interest, and a better understanding of this mechanism would be helpful for determining the mechanism of the detonation reaction of explosives. In this study, a ε-CL-20 supercell was constructed, and the thermal decomposition was calculated at different compression densities and temperatures using molecular dynamics simulations based on the ReaxFF-lg reactive force field. We analyzed the effect of density on the main elementary reaction, which consists of the initial reaction and the formation of final products. In addition, we studied the effect of density on the generation of clusters and the reaction kinetics of the thermal decomposition. The results indicate that the initial reaction pathway of the CL-20 molecule is the cleavage of the N–NO2 bond at different densities and that the frequency of N–NO2 bond breakage decreases at high density. As the density increases, clusters easily form and are resistant to decomposition at the later stage of thermal decomposition, which eventually leads to a decrease in the number of final products. Increasing the initial density of CL-20 significantly increases the reaction rate of the initial decomposition but hardly changes the activation energy of the decomposition.


 Please wait while we load your content...
Please wait while we load your content...