Inducing regioselective chemical reactivity in graphene with alkali metal intercalation†
Abstract
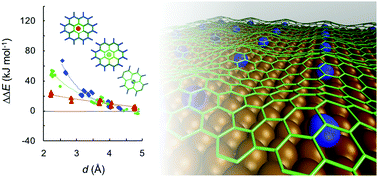
First principles calculations demonstrate that alkali metal atoms, intercalated between metal substrates and adsorbed graphene monolayers, induce localised regions of increased reactivity. The extent of this localisation is proportional to the size of the alkali atom and the strength of the graphene–substrate interaction. Thus, larger alkali atoms are more effective (e.g. K > Na > Li), as are stronger-interacting substrates (e.g. Ni > Cu). Despite the electropositivity of these alkali metal adsorbates, analysis of charge transfer between the alkali metal, the substrate and the adsorbed graphene layer indicates that charge transfer does not give rise to the observed regioselective reactivity. Instead, the increased reactivity induced in the graphene structure is shown to arise from the geometrical distortion of the graphene layer imposed by the intercalated adsorbed atom. We show that this strategy can be used with arbitrary adsorbates and substrate defects, provided such structures are stable, towards controlling the mesoscale patterning and chemical functionalisation of graphene structures.


 Please wait while we load your content...
Please wait while we load your content...