Abstract
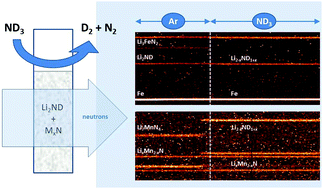
Lithium imide is a promising new catalyst for the production of hydrogen from ammonia. Its catalytic activity has been reported to be significantly enhanced through its use as a composite with various transition metal nitrides. In this work, two of these composite catalysts (with manganese nitride and iron nitride) were examined using in situ neutron and X-ray powder diffraction experiments in order to explore the bulk phases present during ammonia decomposition. Under such conditions, the iron composite was found to be a mixture of lithium imide and iron metal, while the manganese composite contained lithium imide and manganese nitride at low temperatures, and a mixture of lithium imide and two ternary lithium–manganese nitrides (LixMn2−xN and a small proportion of Li7MnN4) at higher temperatures. The results indicate that the bulk formation of a ternary nitride is not necessary for ammonia decomposition in lithium imide–transition metal catalyst systems.


 Please wait while we load your content...
Please wait while we load your content...