Two-dimensional carbon dioxide with high stability, a negative Poisson's ratio and a huge band gap†
Abstract
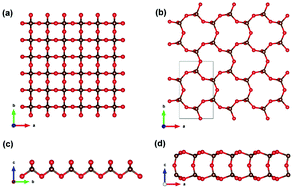
Carbon dioxide (CO2) normally exists in a gaseous molecular state under ambient conditions, while two-dimensional (2D) crystals of CO2 have not been reported yet. In this work, based on density functional theory and the particle swarm optimization method, we unveil two CO2 2D crystals with space groups of P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) M2 and Amm2. Our results show that these structures have excellent thermal, dynamic, and mechanical stability. The new structures are insulators with an indirect or direct band gap, while the indirect band gap can be tuned to be direct with small uniaxial strains. More importantly, the P
M2 and Amm2. Our results show that these structures have excellent thermal, dynamic, and mechanical stability. The new structures are insulators with an indirect or direct band gap, while the indirect band gap can be tuned to be direct with small uniaxial strains. More importantly, the P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) M2 structure has an in-plane negative Poisson's ratio, which is due to the interaction of the lattice symmetry and the local CO4 tetrahedron symmetry. In addition, the Amm2 sheet has a very large electronic band gap (>9 eV), which is the largest in all known 2D materials. Enthalpy curves indicate that these 2D structures may be obtained from the ambient phase of CO2 under high pressure. This work presents new structures of CO2, and because of their excellent performance in terms of stability, mechanical and electronic properties, they potentially have broad applications.
M2 structure has an in-plane negative Poisson's ratio, which is due to the interaction of the lattice symmetry and the local CO4 tetrahedron symmetry. In addition, the Amm2 sheet has a very large electronic band gap (>9 eV), which is the largest in all known 2D materials. Enthalpy curves indicate that these 2D structures may be obtained from the ambient phase of CO2 under high pressure. This work presents new structures of CO2, and because of their excellent performance in terms of stability, mechanical and electronic properties, they potentially have broad applications.


 Please wait while we load your content...
Please wait while we load your content...