Excitation spectroscopic and synchronous fluorescence spectroscopic analysis of the origin of aggregation-induced emission in N,N-diphenyl-1-naphthylamine-o-carborane derivatives†
Abstract
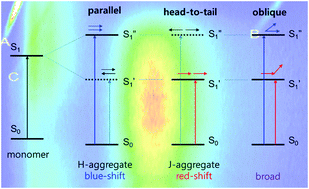
We have synthesised mono-(NpCb) and bis-[(N,N-phenyl-1-naphthylamino)benzo]-o-caboranes (NpCbNp), which show anomalously intense aggregation-induced emission (AIE) at long wavelengths and monomer emission at short wavelengths. The actual concentration of the aggregator in intense AIE is very low, so absorption spectroscopy is unsuitable for detecting small changes in the absorbance. Hence, to understand the aggregation pattern, we employ excitation spectroscopy, since this method has excellent sensitivity in compliance with the emission intensity. Moreover, we carried out synchronous fluorescence spectroscopic measurements to confirm that the aggregator is different from the monomeric species. The excitation spectrum shows distinguishable differences between the AIE and the normal emission. For the triad NpCbNp, the excitation spectrum for the AIE is located at a shorter wavelength than that for the monomeric emission spectrum, which means that the AIE is attributed to the H-type aggregator. On the other hand, for the dyad NpCb, the excitation spectrum for the AIE is observed at an identical wavelength as that for the monomeric species, which indicates that the aggregator is of the oblique type.


 Please wait while we load your content...
Please wait while we load your content...