Suppression of surfaces states at cubic perovskite (001) surfaces by CO2 adsorption†
Abstract
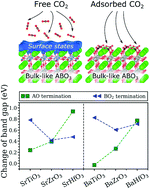
By using first-principles approach, the interaction of CO2 with (001) surfaces of six cubic ABO3 perovskites (A = Ba, Sr and B = Ti, Zr, Hf) is studied in detail. We show that CO2 adsorption results in the formation of highly stable CO3-like complexes with similar geometries for all investigated compounds. This reaction leads to the suppression of the surfaces states, opening the band gaps of the slab systems up to the corresponding bulk energy limits. For most AO-terminated ABO3(001) perovskite surfaces, a CO2 coverage of 0.25 was found to be sufficient to fully suppress the surface states, whereas the same effect can only be achieved at 0.50 CO2 coverage for the BO2-terminated surfaces. The largest band gap modulation among the AO-terminated surfaces was found for SrHfO3(001) and BaHfO3(001), whereas the most profound effect among the BO2-terminated surfaces was identified for SrTiO3(001) and BaTiO3(001). Based on these results and considering practical difficulties associated with measuring conductivity of highly resistive materials, TiO2-terminated SrTiO3(001) and BaTiO3(001) were identified as the most prospective candidates for chemiresistive CO2 sensing applications.


 Please wait while we load your content...
Please wait while we load your content...