Insights on magnesium and sulfate ions’ adsorption on the surface of sodium alumino-silicate hydrate (NASH) gel: a molecular dynamics study
Abstract
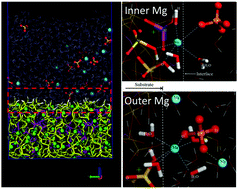
The movement of water and ions in sodium alumino-silicate hydrate gel (NASH) influences the physical and chemical properties of the geopolymer material. In this paper, in order to better understand the structure and dynamics of water and ions in the interfacial region of the NASH gel, molecular dynamics was utilized to model Na2SO4 and MgSO4 solutions (both at 0.44 mol L−1) near the NASH surface. The broken silicate–aluminate surface network, with predominant percentage of randomly connected Q1 and Q2 silicate and aluminate species, provides plenty of non-bridging oxygen sites to accept the H bond from the surface water molecules, contributing toward a strongly adsorbed hydration layer with a thickness of around 5 Å. Consequently, the water molecule in the hydration layer exhibits increased density, increased dipole moment magnitude, orientation preference, and slow diffusivity. In contrast, up to 36.4% of the counter sodium ions, originally caged in the vacancies on the NASH surface, gradually dissociate from the silicate–aluminate skeleton and migrate into the bulk solution, which is consistent with the experimentally observed leaching process of alkali ions in the geopolymer material. In the MgSO4 solution, the magnesium ions—with a smaller ionic radius—penetrate into the silicate–aluminate skeleton vacancy, have 1.8 to 2.5 coordinated solid oxygen atoms, and remain on the NASH surface for a fairly longer time due to the stable Mg–O bonds. Mg species adsorbed on the inner sphere got rooted onto the hydroxyl layer, healing the damaged silicate–aluminate structures and stabilizing the network by inhibiting Na ion immigration into the solution. Mg ions in the outer layer, on average, associated with around one neighboring SO4 ion, forming ionic pairs and accumulating into large Mg–SO4 clusters, to help the immobilization of sulfate ions on the NASH surface.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...