Molecular dynamics study on ions and water confined in the nanometer channel of Friedel's salt: structure, dynamics and interfacial interaction†
Abstract
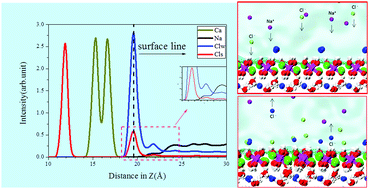
As a promising layered double hydroxide, Friedel's salt has gained popularity. The transport and adsorption behavior of ions and water molecules at the interface is the basis for investigating the durability of concrete, in marine environments in particular. In this paper, the transport behavior of water and ions in the nanopores of Friedel's salt and the adsorption mechanism of the ions were systematically investigated by molecular dynamics. The water molecules share a larger bulk density and good orientations at the interface while the adsorption rate of chloride ions climbs to 66.62%, owing to the desorption of the surface structural anions forming Ca–Clw ion clusters. The time correlation function was employed to examine the stability of the Ca–Clw bonds formed near the Friedel's salt interface. The Ca–Clw bonds were demonstrated to be very stable, implying that the aqueous chloride ion is difficult to desorb once it is adsorbed by the interface. The surface of the ordered Friedel's salt structure could form a hydrated shell to hinder the interaction between sodium ions and oxygen atoms. In addition, Friedel's salt exhibits a poor adsorption capacity for sodium ions since it provides few adsorption sites due to the limited amount of structural chloride ions. After all, the interaction between Friedel's salt and the external environment on the nano scale was explored for a better understanding of the inherent mechanism from a molecular simulation perspective.


 Please wait while we load your content...
Please wait while we load your content...