Macromolecular crowding and the importance of proper hydration for the structure and dynamics of protein solutions†
Abstract
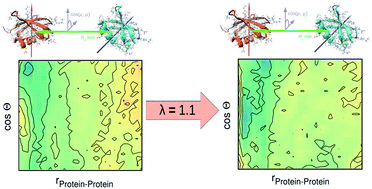
Recent experiments by Weingärtner et al. have given a first hint that dielectric spectroscopy is able to yield a quantitative measure of inter-protein mutual orientation. Therefore, in this computational study, we investigate crowded multi-protein solutions with a special focus on this mutual orientation and its context with dielectric spectroscopy. To the end, existing standard force fields had to be improved by re-scaling the dispersion interaction between protein and water. We find that proper hydration has a strong influence on inter-protein correlations as an enhancement of protein hydration by 10% has a great impact on orientational intermolecular structure. Altogether, the crowding behaviour is improved considerably.


 Please wait while we load your content...
Please wait while we load your content...