Thermodynamic features and enthalpy relaxation in a metal–organic framework glass†
Abstract
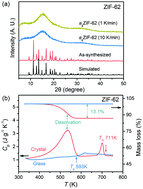
In this work, we explore the thermodynamic evolution in a melt-quenched metal–organic framework glass, formed from ZIF-62 upon heating to the melting point (Tm), and subsequent enthalpy relaxation. The temperature dependence of the difference in Gibbs free energy between the liquid and crystal states of ZIF-62 in the temperature range from the glass transition temperature (Tg) to Tm is found to be weaker than those of other types of glasses, e.g., metallic glasses. Additionally, we find that the stretched exponent of the enthalpy relaxation function in the glass varies significantly (β = 0.44–0.76) upon changing the extent of sub-Tg annealing, compared to metallic and oxide glasses with similar Tgs, suggesting a high degree of structural heterogeneity. Pair distribution function results suggest no significant structural changes during the sub-Tg relaxation in ZIF-62 glass.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...