Photophysical properties of free-base and manganese(iii) N-confused porphyrins†
Abstract
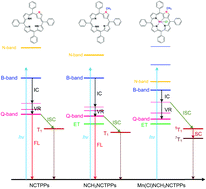
The photophysical properties of N-confused 5,10,15,20-tetraphenyl porphyrin derivatives have been studied using steady-state and time-resolved spectroscopic techniques. The peripherally substituted N-confused 5,10,15,20-tetraphenyl free-base porphyrins (NCTPPs) show stronger B-band absorptions in DCM than in DMAc, while much stronger emissions have been observed in DMAc, which may be due to the shorter times (τIC) of internal conversion from the B-band to the Q-band. The Q-band spectral structures of NCTPPs in DCM are significantly different from those in DMAc. The introduction of ortho-OCH3 results in the strongest emission in both DCM and DMAc and significant fluorescence after N-methylation even though the emissions of other N-methyl complexes are quenched. The N-methylation of NCTPPs leads to a larger τIC and shorter emission lifetime. The excited-state dynamics of manganese(III) N-confused porphyrins (Mn(Cl)NCH3NCTPPs) are influenced by both peripheral substituents and manganese(III) metal ion, and exhibit ultrafast intersystem crossing processes.


 Please wait while we load your content...
Please wait while we load your content...