Hydrogen storage on volleyballene†
Abstract
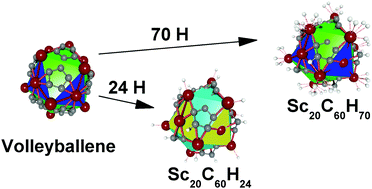
This study is devoted to the hydrogenation of the recently predicted volleyballene (Sc20C60) compound. The hydrogenation produces structures with 24 and 70 H atoms, respectively. Twelve of the 20 available Sc atoms and C5 rings are found as preferred sites for hydrogenation. DFT calculations attest a H2 uptake capacity of 4.20 wt% for the heavily hydrogenated structure (Sc20C60H70), and a corrected −0.11 eV/H2 adsorption energy value. Calculated entalphy (negative) and entropy (positive) values assure that the hydrogenation reaction of volleyballene can be achieved at ambient temperature. The heavily hydrogenated structure holds a 1.1 eV HOMO–LUMO gap value and it does not feature imaginary frequencies attesting its experimental convenience.


 Please wait while we load your content...
Please wait while we load your content...