Insights into the complex interaction between hydrophilic nanoparticles and ionic surfactants at the liquid/air interface
Abstract
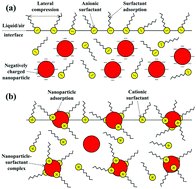
Combinations of nanoparticles and surfactants have been widely employed in many industrial processes, i.e., boiling and condensation in heat transfer and hydraulic fracturing in shale oil and gas production, etc. However, the underlying mechanism for various phenomena resulting from the addition of nanoparticles into the surfactant solutions is still unclear. For instance, there are contradictory conclusions from the literature regarding the variations of surface tension upon the addition of nanoparticles into surfactant solutions. In this work, the dominating factors determining if the surface activity of the surfactant solution will increase or conversely decrease when adding certain kinds of nanoparticles have been investigated. Two typical hydrophilic nanoparticles, SiO2 and TiO2 with anionic or cationic surfactants, respectively, have been considered. The surface tension has been measured in a wide range of nanoparticle and surfactant concentrations. It was found that the surface tension of the ionic surfactant solution can be further reduced only if nanoparticles of the same charge were added. For instance, a system containing 0.25 CMC SDS and 1 wt% SiO2 behaves similar to a 0.34 CMC SDS-only solution. Interestingly, the observed synergistic effect is found to be more significant if the surfactant concentration is much lower than its CMC for a given nanoparticle content. Moreover, the effect is perfectly reversible. When the nanoparticles were separated from the system, the surface tension values recovered fully to that of the pure surfactants. If nanoparticles of opposite charge were added, however, the surface tension of the surfactant solution increased. Zeta potential measurement and centrifugal treatment have been employed to reveal the interplay between nanoparticles and surfactants and the adsorption behavior of their assemblies at the liquid/air interface. Based on the experimental outcomes, a possible physical mechanism was proposed. It was concluded that the electrostatic repulsion between surfactant molecules and nanoparticles should be the dominant factor responsible for the observed reversible synergistic effect. Our study is expected to contribute to a better understanding of the interfacial phenomenon in nanoparticle–surfactant complex systems.


 Please wait while we load your content...
Please wait while we load your content...