Maximum bonding fragment orbitals for deciphering complex chemical interactions†
Abstract
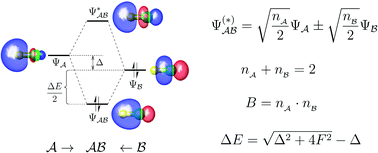
An optimal set of fragment orbitals is proposed that offers a simple and general way to describe complex bonding situations. The idea is based on the unique decomposition of the total bond order between fragments. Each resulting component corresponds to the bonding between a pair of optimal fragment orbitals. These pairs further form a set of exactly doubly occupied bond orbitals, which fully describes the chemical bonding between two fragments. Thereby, complex bonding interactions can be separated into a few well-defined pairwise orbital interactions. A remarkable feature of the theory is that one can build up a quantitative and clearly correlated orbital interaction diagram. Explicit analytical expressions are also presented for bond orders, bond polarities, occupancies, and orbital interaction energies, providing new insights into the bond order concept and the fundamental principles of molecular orbital theory. Through illustrative examples, this new approach is shown to be a simple and powerful tool for analyzing different kinds of chemical interactions, including covalent, ionic and noncovalent bonding.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...