Solvent dependent morphology and 59Co internal field NMR study of Co-aggregates synthesized by a wet chemical method
Abstract
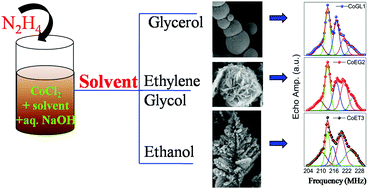
Different shapes of Co-aggregates were synthesized via reduction of a Co salt (CoCl2·6H2O) by chemical precipitation using glycerol, ethylene glycol and ethanol as solvents. The effect of solvent on the morphology, fcc or hcp phase-content and the magnetic properties of the synthesized samples were investigated. The Co-aggregates synthesized using glycerol have a dense spherical shape and high saturation magnetization (MS), whereas ethylene glycol leads to formation of flower-shaped spherical aggregates through loose packing of smaller plate-like particles which have a moderate MS value. When ethanol was used as a solvent, a dendritic (leaf like)-shape of the aggregates with the lowest MS value was obtained. The formation of the obtained morphology of the aggregates was explained based on the size of the solvent molecule, the viscosity of the solvent and the number of polar groups (–OH) present in the solvent molecules. The magnetic domain state and domain wall dynamics of all the Co-samples were investigated using 59Co Internal Field Nuclear Magnetic Resonance (IFNMR) spectroscopy at RT and at 77 K. Through the IFNMR spectroscopy, the presence of gain boundaries, single domain particles and multi-domain particles/aggregates with domain walls associated with fcc and hcp phases were identified and quantified. We observed that the use of ethanol facilitates formation of a higher amount of hcp phase in the sample than the use of glycerol or ethylene glycol.


 Please wait while we load your content...
Please wait while we load your content...