17O and 1H NMR spectral parameters in isolated water molecules
Abstract
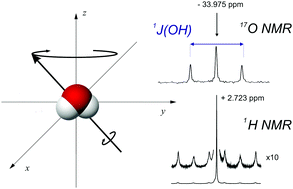
Small amounts of water enriched in oxygen-17 were studied by 17O and 1H NMR in binary gaseous mixtures with Xe, Kr, CHF3 and CH3F and CO2. The distinct linear dependences of 17O and 1H chemical shifts and 1J(17O,1H) spin–spin coupling on the density of every gas solvent were measured. After the extrapolation of experimental results to zero density the relevant parameters in the isolated H217O molecule were determined. The same procedure was applied for H216O when its proton chemical shift was analyzed but the secondary isotope effect in the 1H shielding of H217O and H216O molecules was too small for detection. As shown, all the intermolecular effects in nuclear magnetic shielding are negative and these effects are more significant for 17O nuclei than for protons. It is consistent with the appropriate gas-to-liquid shifts of water which also indicate deshielding effects for both the investigated nuclei. On the other hand, the 1J0(17O,1H) coupling constant in H217O, which is completely free from intermolecular interactions, considerably differs from the 1J(17O,1H) experimental values obtained for water in liquid solutions. The present experimental data of the isolated H217O molecule are compared with selected results of shielding and spin–spin coupling calculations available from the literature and with the recent experimental data for a water molecule encapsulated in the C60 fullerene. Additionally, on the basis of actual results the magnetic dipole moment of the 17O nucleus is revalued for greater accuracy.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...