Ab initio investigation of the ground and excited states of MoO+,2+,− and their catalytic strength on water activation†
Abstract
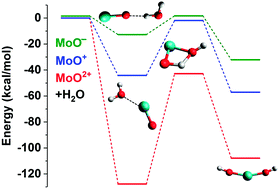
The charged molybdenum monoxides, MoO+,2+,− were studied by multireference configuration interaction and coupled cluster calculations in conjunction with large basis sets. Full potential energy curves were constructed and bonding patterns were proposed for several low-lying electronic states of the three species. Our numerical results involve accurate equilibrium bond lengths, harmonic vibrational frequencies, anharmonicities, excitation energies, and binding energies. This is the first high-level theoretical investigation and our results compare favorably with the limited existing experimental data. Nine states of MoO2+ are bound with respect to the lowest energy fragments Mo+ + O+, while MoO− has five bound electronic states with respect to MoO + e−. Energetics, including activation energies, are given for the reaction between the lowest lying electronic states of the titled species and water. It is shown that MoO− is clearly more efficient at activating an OH bond.


 Please wait while we load your content...
Please wait while we load your content...