Conformationally-restricted bicarbazoles with phenylene bridges displaying deep-blue emission and high triplet energies: systematic structure–property relationships†
Abstract
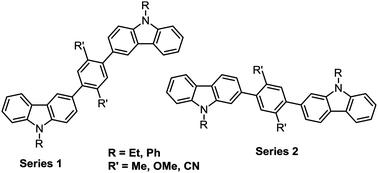
The synthesis is reported of twelve new symmetrical carbazole dimers in which the carbazole units are linked via 1,4-phenylene spacers. There are two distinct series of compounds based on the position on the carbazole ring where the phenylene spacer is attached: this is either at carbazole C(3) (series 1a–1f) or at C(2) (series 2a–2f). The central phenylene ring is substituted with either two methyl, two methoxy or two cyano substituents which impart an intramolecular torsional angle between the phenylene and carbazole rings, thereby limiting the extent of π-conjugation between the carbazole units, and raising the triplet energies of the molecules to ET 2.6–3.0 eV, as determined from their phosphorescence spectra at 80 K. Structure–property relationships were studied by UV-vis and fluorescence spectroscopy, cyclic voltammetry and theoretical calculations. A notable observation is that substitution at the 2-position of carbazole (linear conjugation) exerts control over the position of the HOMO, while substitution at the 3-position of carbazole (meta conjugation) allows greater control over the LUMO. X-ray crystal structures are reported for two of the bicarbazoles. Compound 2d is shown to be a suitable host for the sky-blue emitter FIrpic in PhOLEDs, with improved device performance compared to CBP as host.


 Please wait while we load your content...
Please wait while we load your content...