Unexpected protonation state of Glu197 discovered from simulations of tacrine in butyrylcholinesterase
Abstract
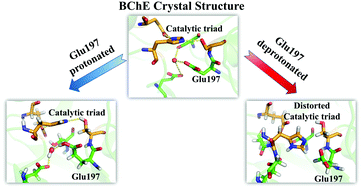
Butyrylcholinesterase (BChE) has been actively involved in drug discoveries from many fields for decades. In the crystal structure of the BChE–tacrine complex, there is an unanticipated formyl-proline molecule resolved very close to tacrine, raising an essential question on how reliable it is to apply the binding pose in a crystal structure to analyze related experimental observations, in which no formyl-proline is actually involved. In this study, by performing a series of 100 ns molecular dynamics simulations, we demonstrate that it is safe to employ the structural information from this crystal structure to analyze related experimental observations. Surprisingly, Glu197 needs to be protonated to have the structures simulated appropriately. It should be noted that Glu197 has been commonly considered as deprotonated in diverse analyses due to its low pKa in aqueous solution, for which some interpretations are inconsistent or unclear. Our further investigation shows that the protonated Glu197 plays a very important role in preserving His438 within the catalytic triad through stabilizing a highly conserved water molecule. Interestingly, the catalytic triad and Glu197 have been long recognized for possibly deviating largely from the crystal structure, which might be catalytically deficient and is generally considered to result from the difference between the crystal and aqueous environment. Herein, our results suggest that the large deviations of the catalytic triad and Glu197 from the crystal structure are caused by the inappropriate protonation state of Glu197. This finding shall provide an important clue that has been long missing for a better understanding of BChE-related puzzles or even reconsideration of some BChE-catalyzed reaction mechanisms.


 Please wait while we load your content...
Please wait while we load your content...