Fundamental limitation of electrocatalytic methane conversion to methanol
Abstract
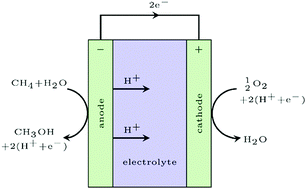
The electrochemical oxidation of methane to methanol at remote oil fields where methane is flared is the ultimate solution to harness this valuable energy resource. In this study we identify a fundamental surface catalytic limitation of this process in terms of a compromise between selectivity and activity, as oxygen evolution is a competing reaction. By investigating two classes of materials, rutile oxides and two-dimensional transition metal nitrides and carbides (MXenes), we find a linear relationship between the energy needed to activate methane, i.e. to break the first C–H bond, and oxygen binding energies on the surface. Based on a simple kinetic model we can conclude that in order to obtain sufficient activity oxygen has to bind weakly to the surface but there is an upper limit to retain selectivity. Few potentially interesting candidates are found but this relatively simple description enables future large scale screening studies for more optimal candidates.

 Please wait while we load your content...
Please wait while we load your content...