Multiphoton-gated cycloreversion reaction of a fluorescent diarylethene derivative as revealed by transient absorption spectroscopy
Abstract
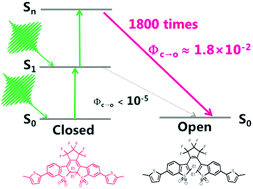
The one- and two-photon cycloreversion reactions of a fluorescent diarylethene derivative with oxidized benzothiophene moieties were investigated by means of ultrafast laser spectroscopy. Femtosecond transient absorption spectroscopy under the one-photon excitation condition revealed that the excited closed-ring isomer is simply deactivated into the initial ground state with a time constant of 2.6 ns without remarkable cycloreversion, the results of which are consistent with the very low cycloreversion reaction yield (<10−5) under steady-state light irradiation. On the other hand, an efficient cycloreversion reaction was observed under irradiation with a picosecond laser pulse at 532 nm. The excitation intensity dependence of the cycloreversion reaction indicates that a highly excited state attained by the stepwise two-photon absorption is responsible for the marked increase of the cycloreversion reaction, and the quantum yield at the highly excited state was estimated to be 0.018 from quantitative analysis, indicating that the reaction is enhanced by a factor of >1800.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...