On the mechanism of spontaneous thiol–disulfide exchange in proteins†
Abstract
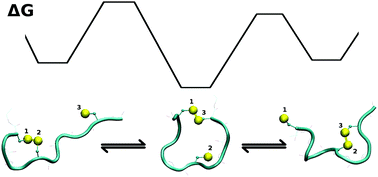
The thiol–disulfide exchange reaction in model systems and small peptides was investigated by means of a combined QM/MM metadynamics scheme. The free energy landscapes of these systems were generated, providing the structures of reactants and products with atomic detail, as well as the heights of free energy barriers (or, activation energies) opposing the spontaneous exchange. A QM/MM scheme with purely classical water turned out to be an efficient and accurate compromise solution. The calculations yielded the expected symmetric trisulfide transition state at S–S distances of 2.7 Å, interestingly, with a slight deviation from linearity at an S–S–S angle of 165°. The structure of the transition state as well as the free energy barrier were very similar for the intramolecular thiol–disulfide reactions in model peptides. While CXC disulfide bonds were found sterically unfavorable, CXXC were favored over longer-range disulfide bonds along the peptide backbone, in line with the high abundance of CXXC motifs in redox proteins.


 Please wait while we load your content...
Please wait while we load your content...