Assessing the electron transfer and oxygen mass transfer of the oxygen reduction reaction using a new electrode kinetic equation†
Abstract
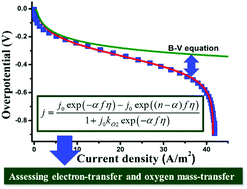
The oxygen reduction reaction (ORR), which is widely employed for energy harvesting and environmental purification, requires an electrode kinetic equation for assessing the electron transfer (ET) and oxygen mass transfer (OMT). Herein, we establish a new kinetic equation in conjunction with the ET kinetics and OMT flux, creating a parameter (kO2) characterizing the effect of OMT. This equation allows for the nonlinear fitting of polarizations in full scale and outputs reliable parameters, including α (ET coefficient), j0 (exchange current density) and kO2. The performance is superior to the Tafel equation by outputting reliable values of α and j0, and covers the function of the Koutecky–Levich equation to calculate the electron transfer number (n) as well. Furthermore, by means of kO2, the assessment of OMT becomes available, disclosing the facilitating effect brought about by the porous structure on the ORR rate. Consequently, the new equation provides a reliable and facile approach for assessing the performance of electrode reaction systems and electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...