Counterion binding alters surfactant self-assembly in deep eutectic solvents†
Abstract
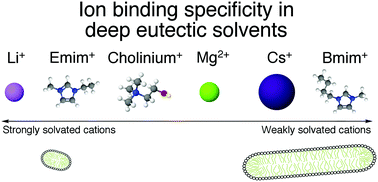
Micellisation of surfactants in deep eutectic solvents has been recently demonstrated to provide a controllable way to modify micelle morphology. Ion-pair interactions between the solvent and the surfactant headgroup were identified as affecting the micellisation by modifying the charge density of the micelle. Here we explore the micellisation of dodecylsulfate surfactants with different counterions (Li+, Cs+, Mg2+, Bmim+, Emim+, cholinium+) dissolved in two deep eutectic solvents: choline chloride:urea and choline chloride:glycerol. Surface tension results show a solvent and counterion dependence of the CMC of the surfactants. Small-angle neutron scattering was subsequently used to investigate the morphology of the micelles formed. The results show that the elongation of the micelles is strongly dependent on the solvent, showing more elongated aggregates in choline chloride:urea than in choline chloride:glycerol. The morphology of micelles in DES was also found to depend on the counterion, where the affinity of binding showed similarities to that in water.


 Please wait while we load your content...
Please wait while we load your content...