Metathesis of Mg2FeH6 and LiNH2 leading to hydrogen production at low temperatures
Abstract
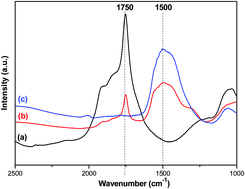
Mg2FeH6 with a purity of up to 94.5 wt% was synthesized and its interaction with LiNH2 was investigated in this study. It was found that Li4FeH6, normally synthesized by hydriding a mixture of LiH and Fe at 700 °C and 5.5 GPa H2 pressure, can be formed via ball-milling Mg2FeH6 and LiNH2 under ambient conditions following the reaction of Mg2FeH6 + 4LiNH2 → Li4FeH6 + 2Mg(NH2)2, ΔH = −92.8 kJ mol−1. The formation of Li4FeH6 was confirmed by XRD, FTIR and Mössbauer spectroscopic characterization. Li4FeH6 further reacts with 2Mg(NH2)2 releasing ca. 4.8 wt% H2 at 225 °C and reabsorbing 3.7 wt% H2 at 200 °C and 50 bar H2 pressure. Mg(NH2)2, LiH and Fe are the hydrogenated products.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...