Chiral polymorphism in the self-assemblies of achiral molecules induced by multiple hydrogen bonds†
Abstract
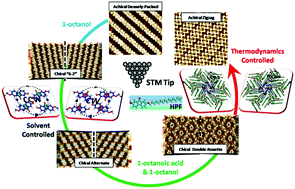
Owing to a wide range of applications within the areas such as chiral sensors, enantiomeric resolution, and asymmetric catalysis, understanding chiral adsorption phenomena at the interface is thereby of great importance. In particular, the role of multiple hydrogen bonds in inducing chiral diversiform morphologies has never been systematically investigated. Herein, by delicate control of the volume ratio of 1-octanoic acid and 1-octanol as the mixed solvent, a series of self-assembled nanostructures of 2-hydroxyl-7-pentadecyloxy-fluorenone (HPF) were sequentially fabricated, including the achiral densely-packed pattern, the chiral “6-2” pattern, the chiral alternate pattern, and the chiral double-rosette pattern. Eventually, those patterns would evolve into an achiral and thermodynamically favored zigzag pattern. Based on DFT calculations, we demonstrate that the stabilities of diversiform morphologies originate from different hydrogen bonding and molecular packing densities. In addition, quantum theory of atoms in molecule (QTAIM) analysis is further applied to interpret the nature of these hydrogen bonds.


 Please wait while we load your content...
Please wait while we load your content...