Anisotropic and amphoteric characteristics of diverse carbenes†
Abstract
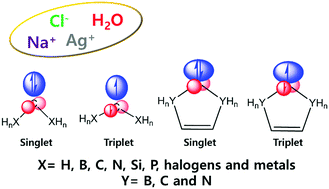
Despite its key importance in carbene chemistry, the amphoteric (i.e., both nucleophilic and electrophilic) behavior of the divalent carbon atom (:C) in carbenes is not well understood. The electrostatic potential (EP) around :C is often incorrectly described by simple isotropic atomic charges (particularly, as in singlet CF2); therefore, it should be described by the multipole model, which can illustrate both negative and positive EPs, favoring the positively and negatively charged species that are often present around :C. This amphotericity is much stronger in the singlet state, which has more conspicuous anisotropic charge distribution than the triplet state; this is validated by the complexation structures of carbenes interacting with Na+, Cl−, H2O, and Ag+. From the study of diverse carbenes [including CH2, CLi2/CNa2, CBe2/CMg2, CF2/CCl2, C(BH2)2/C(AlH2)2, C(CH3)2/C(SiH3)2, C(NH2)2/C(PH2)2, cyclic systems of C(CH2)2/C(CH)2, C(BHCH)2, C(CH2CH)2/C(CHCH)2, and C(NHCH)2/C(NCH)2], we elucidate the relationships between the electron configurations, electron accepting/donating strengths of atoms attached to :C, π conjugation, singlet–triplet energy gaps, anisotropic hard wall radii, anisotropic electrostatic potentials, and amphotericities of carbenes, which are vital to carbene chemistry. The (σ2, π2 or σπ) electronic configuration associated with :C on the :CA2 plane (where A is an adjacent atom) in singlet and triplet carbenes largely governs the amphoteric behaviors along the :C tip and :C face-on directions. The :C tip and :C face-on sites of σ2 singlet carbenes tend to show negative and positive EPs, favoring nucleophiles and electrophiles, respectively; meanwhile, those of π2 singlet carbenes, such as very highly π-conjugated 5-membered cyclic C(NCH)2, tend to show the opposite behavior. Open-shell σπ singlet (such as highly π-conjugated 5-membered cyclic C(CHCH)2) and triplet carbenes show less anisotropic and amphoteric behaviors.


 Please wait while we load your content...
Please wait while we load your content...