The role of electronegativity on the extent of nitridation of group 5 metals as revealed by reactions of tantalum cluster cations with ammonia molecules†
Abstract
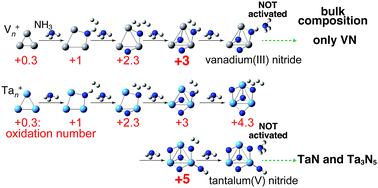
Reactions of the free tantalum cation, Ta+, and tantalum cluster cations, Tan+ (n = 2–10), with ammonia are presented. The reaction of the monomer cation, Ta+, with two molecules of NH3 leads to the formation of TaN2H2+ along with release of two H2 molecules. The dehydrogenation occurs until the formal oxidation number of the tantalum atom reaches +5. On the other hand, all the tantalum cluster cations, Tan+, react with two molecules of NH3 and form TanN2+ with the release of three H2 molecules. Further exposure to ammonia showed that TanNmH+ and TanNm+ are produced through successive reactions; a pure nitride and three H2 molecules are formed for every other NH3 molecule. The nitridation occurred until the formal oxidation number of the tantalum atoms reaches +5 as in the case of TaN2H2+ in contrast to other group 5 elements, i.e., vanadium and niobium, which have been reported to produce nitrides with lower oxidation states. The present results on small gas-phase metal-nitride clusters show correlation with their bulk properties: tantalum is known to form bulk nitrides in the oxidation states of either +5 (Ta3N5) or +3 (TaN), whereas vanadium and niobium form nitrides in the oxidation state of +3 (VN and NbN). Along with DFT calculations, these findings reveal that nitridation is driven by the electron-donating ability of group 5 elements, i.e., electronegativity of the metal plays a key role in determining the composition of the metal nitrides.
- This article is part of the themed collections: New Frontiers in Indian Research and New Frontiers in Indian Research


 Please wait while we load your content...
Please wait while we load your content...