Inorganic mixed phase templated by a fatty acid monolayer at the air–water interface: the Mn and Mg case†
Abstract
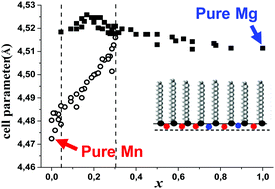
We studied by means of Grazing Incidence X-ray Diffraction (GIXD) coupled with X-ray fluorescence spectroscopy the structure of a behenic acid monolayer spread at the surface of Mg2+/Mn2+ mixed aqueous solutions. For the pure Mg2+ and Mn2+ aqueous solutions, the cations induce at the surface different 2D lattice superstructures of the organic monolayer. These superstructures correspond to an inorganic organized monolayer anchored to the hydrophilic group of the ordered behenic acid monolayer. Among the various diffraction peaks, we focused on those characteristics of the behenic acid oblique cell. As the Mg2+ mole fraction x increases in the Mg2+/Mn2+ mixed subphase, a continuous evolution of the oblique cell parameters is observed indicating the insertion of Mg2+ cations in the Mn2+ ordered monolayer. Then, a further increase leads to the appearance of a coexistence between two oblique surface phases. The cell parameters of both phases evolve continuously along the x range of the transition until a single Mg-rich ordered phase is detected. However, although the intensities of the peaks in the coexistence region are in agreement with a first-order phase transition, the cell parameters evolve simultaneously. Considering a thermodynamics analysis, this evidences that, apart from the concentration, another unidentified intensive parameter is varying. We suggest that it is the ionic strength, which appears to be strongly related to the concentrations.


 Please wait while we load your content...
Please wait while we load your content...