Aggregation-induced emission and the working mechanism of 1-benzoyl and 1-benzyl pyrene derivatives†
Abstract
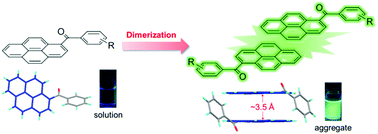
Over the past decade, research studies on solid-state luminescent materials featuring aggregation-induced emission (AIE) have achieved great success. It has been proved that lots of planar ACQ (aggregation-caused quenching) chromophores can be converted to AIE luminogens (AIEgens) by combining with an AIE-active unit such as tetraphenylethene (TPE). In this work, we present a new method to create AIE luminogens just by introducing benzoyl or benzyl to a planar chromophore, pyrene. The generated 1-benzoyl and 1-benzyl pyrene derivatives exhibit weak emission when molecularly dissolved in good solvents but strong emission from pyrene dimers when aggregated in poor solvent or the solid state. Their crystal structure analysis and theoretical calculations are performed to depict the working mechanism of these new AIEgens. The results show that the structural rigidification of these 1-benzoyl pyrene derivatives is the major cause for their AIE effect. This new AIE system along with a clear working mechanism will contribute to the development of AIE-related functional materials and theories.


 Please wait while we load your content...
Please wait while we load your content...