Anharmonicity in a double hydrogen transfer reaction studied in a single porphycene molecule on a Cu(110) surface
Abstract
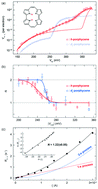
Anharmonicity plays a crucial role in hydrogen transfer reactions in hydrogen-bonding systems, which leads to a peculiar spectral line shape of the hydrogen stretching mode as well as highly complex intra/intermolecular vibrational energy relaxation. Single-molecule study with a well-defined model is necessary to elucidate a fundamental mechanism. Recent low-temperature scanning tunnelling microscopy (STM) experiments revealed that the cis ↔ cis tautomerization in a single porphycene molecule on Cu(110) at 5 K can be induced by vibrational excitation via an inelastic electron tunnelling process and the N–H(D) stretching mode couples with the tautomerization coordinate [Kumagai et al. Phys. Rev. Lett. 2013, 111, 246101]. Here we discuss a pronounced anharmonicity of the N–H stretching mode observed in the STM action spectra and the conductance spectra. Density functional theory calculations find a strong intermode coupling of the N–H stretching with an in-plane bending mode within porphycene on Cu(110).


 Please wait while we load your content...
Please wait while we load your content...