Concerted motions and large-scale structural fluctuations of Trichoderma reesei Cel7A cellobiohydrolase†
Abstract
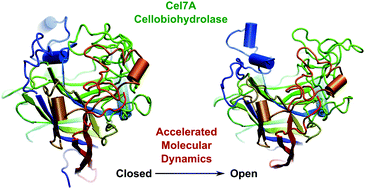
Cellobiohydrolases (CBHs) are key enzymes for the saccharification of cellulose and play major roles in industrial settings for biofuel production. The catalytic core domain of these enzymes exhibits a long and narrow binding tunnel capable of binding glucan chains from crystalline cellulose and processively hydrolyze them. The binding cleft is topped by a set of loops, which are believed to play key roles in substrate binding and cleavage processivity. Here, we present an analysis of the loop motions of the Trichoderma reesei Cel7A catalytic core domain (TrCel7A) using conventional and accelerated molecular dynamics simulations. We observe that the loops exhibit highly coupled fluctuations and cannot move independently of each other. In the absence of a substrate, the characteristic large amplitude dynamics of TrCel7A consists of breathing motions, where the loops undergo open-and-close fluctuations. Upon substrate binding, the open-close fluctuations of the loops are quenched and one of the loops moves parallel to the binding site, possibly to allow processive motion along the glucan chain. Using microsecond accelerated molecular dynamics, we observe large-scale fluctuations of the loops (up to 37 Å) and the entire exposure of the TrCel7A binding site in the absence of the substrate, resembling an endoglucanase. These results suggest that the initial CBH-substrate contact and substrate recognition by the enzyme are similar to that of endoglucanases and, once bound to the substrate, the loops remain closed for proper enzymatic activity.


 Please wait while we load your content...
Please wait while we load your content...