Molecular structure and interactions of water intercalated in nickel hydroxide
Abstract
The structure and properties of α-Ni(OH)2 containing water and nitrate have been investigated computationally. The adsorption of water molecules on the Ni(OH)2 surface is also investigated to provide insight into the nature of the water–Ni(OH)2 interactions. The spectroscopic and dynamical behaviour of the intercalated species has been characterized and used to explain experimental findings reported for this material. The results presented here indicate that the water molecules interact non-covalently with Ni(OH)2, with a binding energy that is comparable in magnitude with that of the water dimer hydrogen bond. The presence of the intercalated species increases the distance between the Ni(OH)2 layers such that the interlayer interactions are negligible. The weakening of the interlayer interactions facilitates the horizontal displacement of the layers relative to one another, providing a possible origin for stacking faults observed in α-Ni(OH)2. Comparison of the vibrational frequencies calculated here with the experimental spectra confirms that α-Ni(OH)2 containing only water molecules can be synthesized. The structures of the water molecules intercalated in α-Ni(OH)2 were found to be analogous to those absorbed in γ-NiOOH, while the water–layer interactions are stronger in γ-NiOOH. The results presented here characterize the structure and interactions of water intercalated in nickel hydroxides and also provide insights into the effects of intercalated water on the properties of layered metal hydroxides.
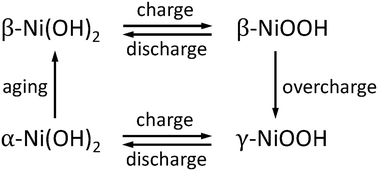


 Please wait while we load your content...
Please wait while we load your content...