Role of the (H2O)n (n = 1–3) cluster in the HO2 + HO → 3O2 + H2O reaction: mechanistic and kinetic studies†
Abstract
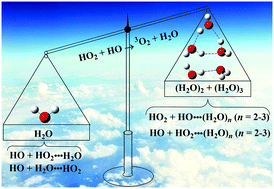
To study the catalytic effects of (H2O)n (n = 1–3), the mechanisms of the reaction HO2 + HO → 3O2 + H2O without and with (H2O)n (n = 1–3) have been investigated theoretically at the CCSD(T)/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ level of theory, coupled with rate constant calculations using the conventional transition state theory. Our results show that upon incorporation of (H2O)n (n = 1–3) into the channel of H2O + 3O2 formation, two different reactions, i.e. HO + HO2⋯(H2O)n (n = 1–3) and HO2 + HO⋯(H2O)n (n = 1–3), have been observed, and these two reactions are competitive with each other. The catalytic effects of (H2O)n (n = 1–3) mainly arise from the contribution of a single water vapor molecule; this is because the effective rate constants with water are respectively larger by 2–3 and 3–4 orders of magnitude than those of the reactions with (H2O)2 and (H2O)3. Furthermore, the catalytic effects of the water monomer mainly arise from the H2O⋯HO2 + HO reaction, and the enhancement factor of this reaction is obvious within the temperature range of 240.0–425.0 K, with the branching ratio (k′(RW)/ktot) of 17.27–80.77%. Overall, the present results provide a new example of how water and water clusters catalyze gas phase reactions under atmospheric conditions.


 Please wait while we load your content...
Please wait while we load your content...