Reactive force-field molecular dynamics study on graphene oxide reinforced cement composite: functional group de-protonation, interfacial bonding and strengthening mechanism†
Abstract
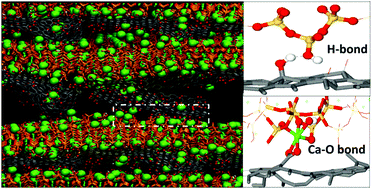
Graphene oxide (GO) reinforced cement nanocomposites open up a new path for sustainable concrete design. In this paper, reactive force-field molecular dynamics was utilized to investigate the structure, reactivity and interfacial bonding of calcium silicate hydrate (C–S–H)/GO nanocomposite functionalized by hydroxyl (C–OH), epoxy (C–O–C), carboxyl (COOH) and sulfonic (SO3H) groups with a coverage of 10%. The silicate chains in the hydrophilic C–S–H substrate provided numerous non-bridging oxygen sites and counter ions (Ca ions) with high reactivity, which allowed interlayer water molecules to dissociate into Si–OH and Ca–OH. On the other hand, protons dissociated from the functional groups and transferred to non-bridging sites in C–S–H, producing carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and Si–OH. The de-protonation degree of the different groups in the vicinity of the C–S–H surface was in the following order: COOH (SO3H) > C–OH > C–O–C. In the GO–COOH sheet, most COOH groups were de-protonated to COO− groups, which enhanced the polarity and hydrophilicity of the GO sheets and formed stable COOCa bonds with neighboring Ca ions. The de-protonated COO− could also accept H bonds from Si–OH in the C–S–H gel, which further strengthened the interfacial connection. On the contrary, in the GO–Oo sheet, only 8% of the epoxy group was stretched open by the Ca ions and transformed to carbonyl group, showing weak polarity and connection with the C–S–H sheet. Furthermore, uniaxial tensile test on different C–S–H/GO models revealed that C–S–H reinforced with GO–COOH and GO–OH had better interfacial cohesive strength and ductility than that observed under tensile loading. Under the reaction force field, the dissociation of water, the proton exchange between the C–S–H and GO structure, and Oc–Ca–Os bond breakage occurred to resist tensile loading. The weakest mechanical behavior observed in the G/C–S–H, GO–Oo/C–S–H and GO–SO3H/C–S–H composites was attributed to the poor bonding, dissociation of functional groups and instability of atoms in the interface region. Hopefully, the molecular-scale strengthening mechanisms could provide a scientific guide for sustainable design of cement composites.
O) and Si–OH. The de-protonation degree of the different groups in the vicinity of the C–S–H surface was in the following order: COOH (SO3H) > C–OH > C–O–C. In the GO–COOH sheet, most COOH groups were de-protonated to COO− groups, which enhanced the polarity and hydrophilicity of the GO sheets and formed stable COOCa bonds with neighboring Ca ions. The de-protonated COO− could also accept H bonds from Si–OH in the C–S–H gel, which further strengthened the interfacial connection. On the contrary, in the GO–Oo sheet, only 8% of the epoxy group was stretched open by the Ca ions and transformed to carbonyl group, showing weak polarity and connection with the C–S–H sheet. Furthermore, uniaxial tensile test on different C–S–H/GO models revealed that C–S–H reinforced with GO–COOH and GO–OH had better interfacial cohesive strength and ductility than that observed under tensile loading. Under the reaction force field, the dissociation of water, the proton exchange between the C–S–H and GO structure, and Oc–Ca–Os bond breakage occurred to resist tensile loading. The weakest mechanical behavior observed in the G/C–S–H, GO–Oo/C–S–H and GO–SO3H/C–S–H composites was attributed to the poor bonding, dissociation of functional groups and instability of atoms in the interface region. Hopefully, the molecular-scale strengthening mechanisms could provide a scientific guide for sustainable design of cement composites.


 Please wait while we load your content...
Please wait while we load your content...